
Even now daily life of Wegovy an injectable prescription fat decline medication that has aided people with being overweight. It should be made use of with a bodyweight loss system and physical activity.
Michael Siluk | UCG | Getty Photographs
Novo Nordisk said Thursday that its blockbuster bodyweight-reduction drug Wegovy could acquire expanded acceptance from the U.S. Foodstuff and Drug Administration inside 6 months.
Main Financial Officer Karsten Munk Knudsen advised CNBC that the Danish pharmaceutical firm had acquired priority evaluation in its software for approval of Wegovy as a treatment method for reducing the possibility of cardiovascular ailment.
A greenlight from the wellness agency could probably increase the insurance plan coverage potential clients of the highly sought-immediately after drug.
“I would say from currently, [the outcome will be] a lot less than 6 months,” Knudsen instructed CNBC’s Julianna Tatelbaum on “Avenue Indications.”
Previously Thursday, Novo Nordisk in its third-quarter earnings declared plans to get expanded approval from the Fda, but shared no timeline. It also claimed record earnings and profits for the period of time on the again of the runaway achievements of its being overweight drug.
Developing wellbeing apps
Late-phase demo facts in August confirmed that Wegovy lowered the hazard of major cardiovascular occasions this sort of as coronary heart assaults or strokes by 20%, in comparison with a placebo.
“The Pick out examine is, in an overweight populace with set up cardiovascular disease, does Wegovy minimize cardiovascular risk? And the solution is, indeed it does, by 20%,” Knudsen said Thursday.
The final results of the intently watched “Find” demo were observed as a boon for Novo Nordisk’s ambitions of relocating further than Wegovy’s impression as a “vanity drug.”
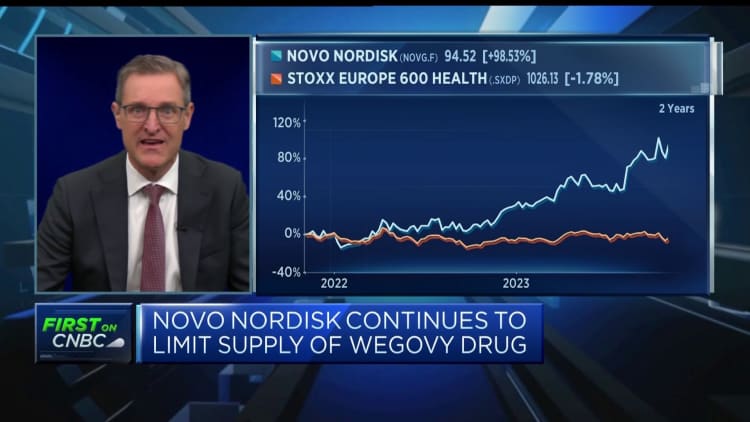
The results presented an included increase for the firm’s stock, which has been on the up this year. Shares had been 1.5% better Thursday morning pursuing its earning’s report above the year to day, shares are virtually 45% bigger.
Knudsen stated the business will current a in depth duplicate of the trial findings in approximately 10 times, immediately after which the Food and drug administration will have six months to deliver its verdict.
He added that acceptance would develop the use situations of the drug, bolstering the possibilities of insurance plan companies shelling out for the procedure. Some insurers have so significantly been unwilling to address the drug — which has a U.S. listing cost of $1,350 a month — only for weight-loss functions.
“The pricing of the products are also a reflection of the benefit that they give to society,” Knudsen stated.
“So the a lot more data we are capable to create, no matter whether it can be on cardiovascular sickness or persistent kidney disorder or other comorbidities, that is of study course element of the over-all price story when we focus on with payers and insurers.”
On the other hand, even with acceptance, provide shortages could keep on to hamper rollout of the drug. Knudsen explained Thursday that the company is continuing to ramp up generation.
“I can guarantee you coming into upcoming yr that we are drastically scaling Wegovy offer,” he claimed.




