
Signage for Eisai Co. at the company’s headquarters in Tokyo, Japan, on Friday, Feb. 3, 2023.
Bloomberg | Bloomberg | Getty Visuals
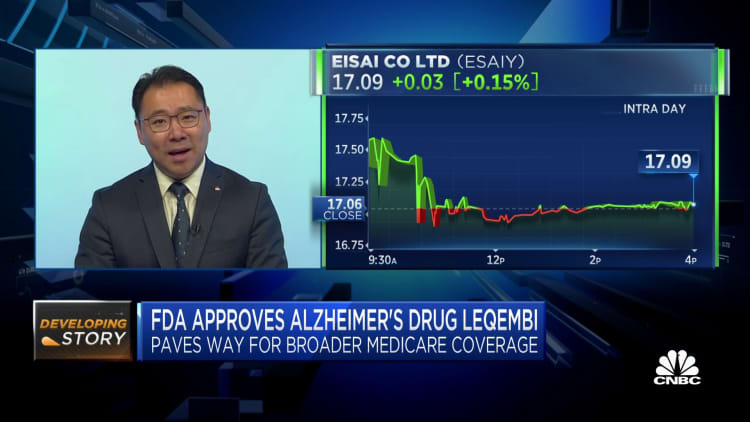
Shares of Japanese pharmaceutical huge Eisai sank Friday following the company’s Alzheimer’s drug was formally accredited by U.S. regulators overnight, prompting queries about trader sentiment surrounding the shift.
Tokyo-outlined shares of Eisai fell by more than 8% at a person stage through Friday trade as traders assessed the U.S. Food and Drug Administration’s acceptance of Leqembi, which is jointly manufactured by its U.S. husband or wife Biogen. Eisai shares shut 4.67% reduced after paring some of its earlier losses.
Leqembi is the initially Alzheimer’s antibody therapy to obtain entire Fda acceptance. It is also the 1st these types of drug that is to obtain broad protection via Medicare.

The closely viewed drug has prompted discussion amongst clinical and marketplace authorities.
University of Cincinnati Faculty of Medicine’s neurologist Dr. Alberto Espay explained to NBC News that the remedy of the drug, precisely the slowing in the progression of the ailment, falls down below the stage that would be thought of “recognizable” to a affected individual.
“The odds for mind inflammation and hemorrhage are far increased than any precise advancement,” Espay, who launched a petition in June calling for the Alzheimer’s procedure to not get complete approval, explained to NBC News.
Eisai U.S. CEO Ivan Cheung refuted this sort of similar fears in an job interview with CNBC’s “Quickly Dollars.”
“This therapy is protected and helpful for Alzheimer’s ailment,” Cheung reported Thursday.
“The benefit possibility profile is effectively established from the substantial late stage clinical trial and we imagine in 12 months a few, about 100,000 persons could be identified and eligible for this essential therapy,” he said.
Naomi Kumagai, senior fairness analyst at Mitsubishi UFJ Morgan Stanley Securities, explained to CNBC through email that a amount of factors were at enjoy in relation to Eisai’s share cost.
She referenced a June 1 announcement from the Centers for Medicare and Medicaid Companies outlining how men and women could accessibility Leqembi the moment Food and drug administration acceptance experienced been granted, as effectively as a optimistic outcome from an advisory committee on June 9.
Given the higher than, “we imagine all the positives are developed in the share selling price, so the shares are down right now, in our vie[w],” Kumagi said.
In order to see a “stable uptake” of the drug, Kumagi highlighted three crucial places, noting that they would not happen in the quick expression. The first would be the approval of a subcutaneous injector formulation, which would present a far more convenient way of administering it. The second and third would be the commercialization of a blood biomarker to detect amyloid beta accumulation, and the reimbursement of these kinds of a blood biomarker.





