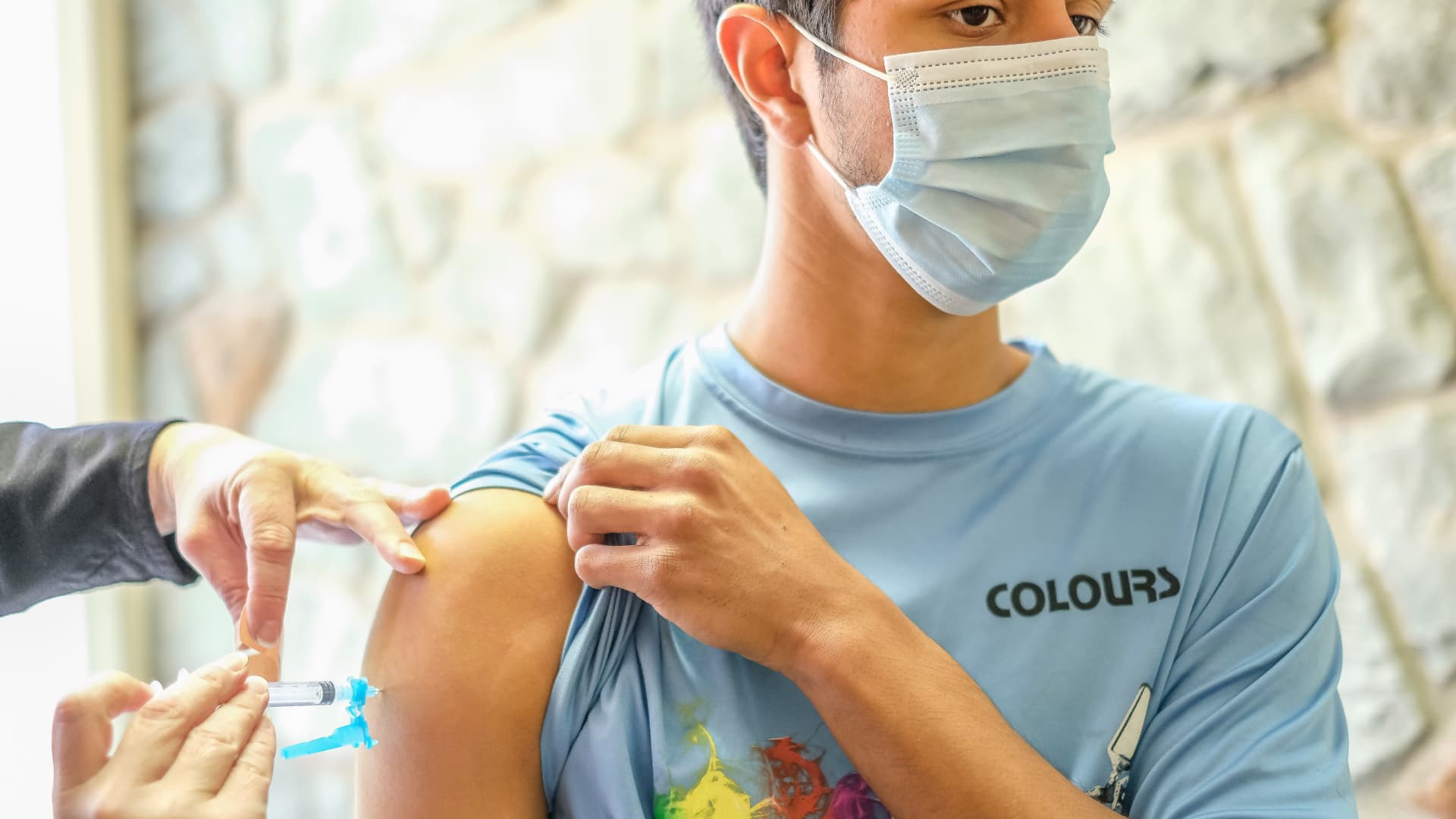
The Centers for Disease Control and Prevention is expected to clear Moderna’s two-dose Covid-19 vaccine for kindergartners through high schoolers for public distribution this week after the agency’s panel of independent vaccine experts unanimously voted Thursday to recommend the shots.
The committee endorsed Moderna’s vaccine for kids ages 6 to 17 after examining its safety and effectiveness during a public meeting. CDC Director Dr. Rochelle Walensky is expected to sign off on the recommendation later Thursday, the final step before pharmacies and doctor’s offices can start administering the shots.
The CDC endorsed Moderna’s vaccines for infants through preschoolers, ages six months to 5-years-old, on Saturday. Vaccinations started this week for that age group.
Moderna’s shots for older kids won’t have an immediate impact on the U.S. vaccination campaign, other than providing parents with another option to choose from. Previously, only Pfizer’s vaccine was authorized for kindergartners through high schoolers, though uptake has been lackluster. Two-thirds of kids ages 5 to 11 and 30% of adolescents ages 12 to 17 haven’t been vaccinated against Covid yet.
More than 600 kids in those age groups have died from Covid during the pandemic and more than 45,000 have been hospitalized, according to the CDC. Nearly 11 million kids ages 5 to 17 have caught Covid during the pandemic.
Kids ages 6 to 11 receive smaller 50 microgram Moderna shots, while adolescents ages 12 to 17 would receive the same dosage as adults at 100 micrograms.
Moderna originally asked the Food and Drug Administration to authorize its vaccine for adolescents ages 12 to 17 more than a year ago, but the regulator held off after other countries raised concern the company’s shots might be associated with a higher risk of heart inflammation, or myocarditis, than Pfizer’s vaccine.
There are no head-to-head comparisons in the U.S. of heart inflammation in kids who get Pfizer’s or Moderna’s shots because Moderna’s vaccine was only authorized for adults until this month. However, comparisons between Pfizer and Moderna shots in young adults appears to show that the rate of myocarditis is slightly higher in Moderna recipients, though data is not consistent across the various U.S. surveillance systems.
“Some evidence suggests that myocarditis and pericarditis risks may be higher after Moderna than after Pfizer. However, the findings are not consistent in all US monitoring systems,” Dr. Tom Shimabukuro, an official at the CDC vaccine safety unit, told the committee.
The available U.S. data on myocarditis among kids ages 6 to 17 is based on side effects reported from Pfizer’s vaccine because Moderna’s shots haven’t been authorized for this age group yet. Pfizer and Moderna’s shots use similar messenger RNA technology.
The CDC has identified 635 cases of myocarditis among children ages 5 to 17 after vaccination out of 54 million Pfizer doses administered. The risk of myocarditis after Pfizer vaccination is highest after the second shot among boys ages 12 to 17. Myocarditis is slightly elevated among boys ages 5 to 11 after the second dose of Pfizer’s vaccine, though it is much lower than adolescents.
Boys ages 16 to 17 reported 75 myocarditis cases per 1 million second Pfizer doses administered while boys ages 12 to 15 reported about 46 myocarditis cases, according to CDC data. Boys ages 5 to 11 reported 2.6 myocarditis cases per million second Pfizer doses administered.
People who have develop myocarditis after vaccination are generally hospitalized for a few days as a precaution before being sent home. The CDC has found that the risk of myocarditis is higher from Covid infection than vaccination. Myocarditis in children is typically caused by viral infections.
The most common side effects among kids ages 6 to 17 during Moderna’s clinical trials were pain at the injection site, fatigue, headache, chills, muscle pain and nausea. There were no confirmed cases of myocarditis during the trials.
It’s unclear how effective the shots will be against the omicron variant. The clinical trials were conducted during periods when other Covid strains were dominant. The shots for adolescents ages 12 to 17 were about 90% effective at preventing illness from the original Covid strain and the alpha variant, while the shots for kids ages 6 to 11 were more than 76% effective at preventing illness from the delta variant, according to the Food and Drug Administration’s review of clinical trial data.
However, the Covid vaccines have trouble fighting the omicron variant, which is now dominant, because it has so many mutations. Third shots have significantly increased protection in other age groups. Moderna is studying booster shots for kids that target omicron with data expected later this summer.







