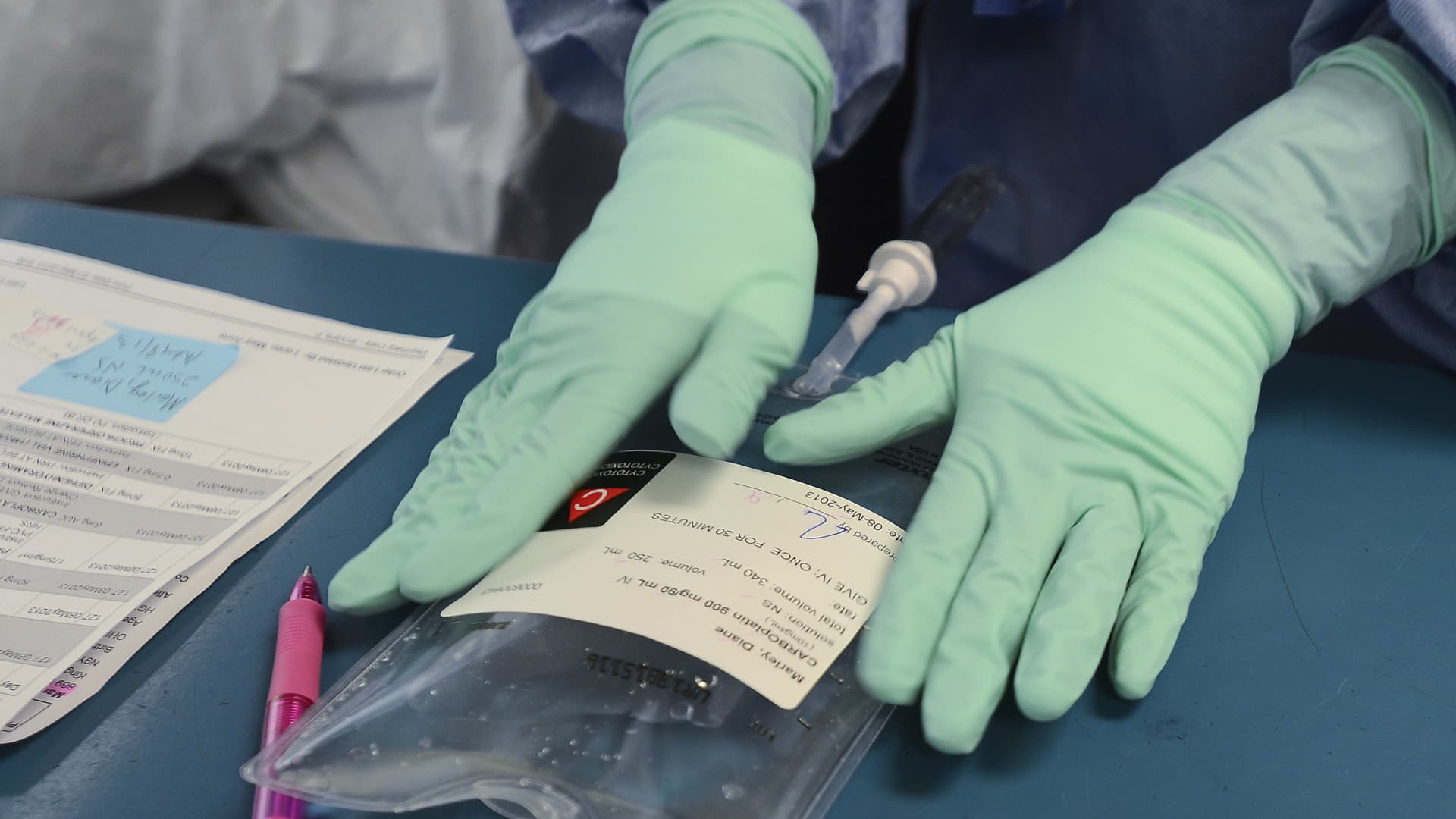
WINDSOR, ON – Could 8: Registered Pharmacy Technician Dawn Deslippe diligently labels Diane’s dose of Carboplatin, one of two chemo prescription drugs she will acquire on this check out. Just about every stage of the system will involve verification from at least two individuals. The healthcare facility now prepares the chemo drugs themselves alternatively than getting them pre-blended.
Diane Marley, 48 is a cancer affected person at Windsor Regional Hospital. She was diagnosed with breast most cancers in December. She is finishing up her chemo routine in the up coming couple of months. She is a single of hundreds of Ontario most cancers people who received diluted chemotherapy in the last year and who are nonetheless going through procedure to defeat the disorder. (Richard Lautens/Toronto Star by using Getty Images)
Richard Lautens | Toronto Star | Getty Images
The Foods and Drug Administration – faced with a countrywide scarcity of much more than a dozen most cancers drugs – is considering allowing the temporary importation of chemotherapy medications from overseas makers that are not at the moment authorized to distribute in the United States, an company spokesperson instructed CNBC.
The Fda did not say which makers would be opportunity candidates for allowing short-term importation of those medicines right until approved producers are ready to meet patients’ demands.
But, “in these instances, we very thoroughly evaluate the abroad merchandise for good quality, generating absolutely sure that its risk-free for U.S. sufferers,” the spokesperson said.
The Fda in the earlier has taken related motion to loosen limitations on imports when confronted with drug shortages. In the summer time of 2022, the Food and drug administration allowed the importation of toddler system from non-company-permitted producers when there was a significant shortage of formulation in the U.S.
The American Society of Medical Oncology anticipates the shortages will continue via June but then subside significantly if the Fda lifts import constraints, according to Dr. Julie Gralow, that group’s main professional medical officer.
“We’re hoping and estimating that once we get by way of the future thirty day period that we will have a more secure provide,” Gralow said.
At least 14 cancer prescription drugs are presently in shorter source throughout the U.S.
But medical doctors at hospitals all over the place say the problem is specifically acute for two medications — cisplatin and carboplatin — due to the fact they are so elementary and commonly applied in cancer treatment method.
The Planet Well being Organization has explained cisplatin and carboplatin are crucial for fundamental wellbeing care.
Intas Prescribed drugs, a person of the greatest makers of all those medications, quickly shut down manufacturing and it is not very clear when the enterprise will resume producing.
Up to 20% of cancer individuals count on platinum-centered chemotherapy drugs this sort of as cisplatin and carboplatin for cure, in accordance to the Countrywide Cancer Institute.
And much more than 100,000 Us citizens were being identified in 2022 with cancers that could be handled with carboplatin or cisplatin, generic drugs that have been on the industry for a long time, the American Culture of Scientific Oncology claims.
Those prescription drugs are applied to handle a vast variety of conditions together with testicular, ovarian, breast, lung, bladder and head-and-neck cancers.
Shortages of the medication have compelled some hospitals to ration the medicines by cutting down doses to lengthen their provide, and to prioritize people who would advantage the most from treatment method.
Some cancer sufferers could die if the shortages are not quickly resolved, medical practitioners explained.
“The lawmakers in the nation require to fully grasp that this is a large challenge at this issue, exactly where except something changes in the future handful of weeks, this can direct to a major national crisis from a individual and health and fitness care standpoint,” stated Dr. Abdul Rafeh Naqash, a medical doctor at the Stephenson Most cancers Middle at the College of Oklahoma.
Naqash explained his facility is on the verge of managing out of carboplatin. He claimed the shortages are a countrywide security challenge that desires to be rapidly addressed.
“Points have been acquiring even worse on the floor. One thing has to materialize and improve right away,” reported Naqash, who specializes in lung most cancers.
He said he recently had to advise a patient that they will not obtain carboplatin owing to the shortage.
These discussions will probable turn out to be extra typical in the coming months if reduction does not come, Naqash said.
Naqash claimed he does not recognize why the U.S. does not have a national stockpile of these medications to fill the hole in emergency circumstances.
Philip Schwieterman, director of oncology and infusion companies at the College of Kentucky wellness system, reported, “If I go in the grocery retailer and I want a kiwi, there are ordinarily kiwis there.”
“It boggles my intellect that if I want some cisplatin, I won’t be able to get cisplatin even however it will save life,” Schwieterman mentioned.
‘A cascading drug shortage’
The cisplatin and carboplatin shortages stem from the short term shutdown of production for the U.S. current market at a plant in India operate by Intas Prescribed drugs.
Intas determined to halt producing right after an Food and drug administration inspection observed a “cascade of failure” in the facility’s top quality control device late past calendar year.
Intas, which is headquartered in Ahmedabad, India, distributes cisplatin and carboplatin in the U.S. by way of its subsidiary, Accord Healthcare.
When the cisplatin shortages commenced in February, lots of sufferers switched to carboplatin, which is regarded as a sister drug, said Marc Phillips, who manages the inpatient pharmacy source chain for WVU Drugs, the premier well being-care system in West Virginia.
That shift has “led into what we take into account a cascading drug lack,” Phillips reported.
“Just one scarcity has now induced a further,” he reported.
Fresenius Kabi, Hikma Pharmaceuticals, Teva and Pfizer generate the drugs, but those people firms have been not able to preserve up with need because the Intas plant went offline.
Intas is working on a prepare with the Food and drug administration to restart production.
But no date has been verified, mentioned business spokesperson Emily King.
When the plant does restart, output will prioritize medicines based on health care requirement, King mentioned.
She famous that the FDA’s drug shortage team and compliance business office have identified carboplatin and cisplatin as a professional medical necessity for the U.S. current market.
The Fda spokesperson stated Intas has begun releasing into the U.S. doses of cisplatin and carboplatin that were being earlier on hold owing to a tests and verification approach.
Ensuring most cancers therapies carry on output
Dr. Karen Knudsen, CEO of the American Most cancers Society, stated the shortages highlight a extensive-standing financial issue in the generic drug market place.
Makers are hesitant to commit more cash in making reduced-expense medications like cisplatin and carboplatin, which would make them vulnerable to shortages when a plant goes down, Knudsen mentioned.
Knudsen fears the U.S. is entering a cycle of most cancers drug shortages if the federal federal government and market do not act with each other to take care of the trouble.
“We have to have it to be economically feasible for producing to be able to create powerful, cost-effective most cancers therapies,” she claimed.
Knudsen claimed demand for these medications will improve in the coming yrs as the population ages mainly because more mature people today are at larger threat for most cancers.
And medicines this sort of as carboplatin and cisplatin use cherished metals – platinum – that are heavily sourced from South Africa and Russia.
The Entire world Platinum Investment Council is forecasting a main deficit of the cherished metallic this 12 months because of in part to disruptions in South Africa caused by an electric power shortage and operational difficulties in Russia owing to sanctions in excess of the Kremlin’s invasion of Ukraine.
Drugmakers are necessary to advise the Fda about production disruptions 6 months in progress or as soon as they are in a position. Knudsen stated the early warning technique doesn’t look to be working successfully.
“The fact that we are sitting here suitable now conversing about this cancer scarcity tells us that the early warning procedure was either not activated early enough, or there are not more than enough companies to be in a position to to defeat the supply chain difficulty,” she stated.
The Fda is functioning with the company to raise offer to meet up with individual need, the company spokesperson mentioned.
A trio of Michigan Democratic lawmakers, Sens. Debbie Stabenow and Gary Peters, Rep. Elissa Slotkin, in a letter very last thirty day period urged Food and drug administration Commissioner Dr. Robert Califf to “use all of its present authorities to mitigate this dire scarcity.”
The letter reported that Congress is functioning on lengthy-expression alternatives to drug shortages, which have been a dilemma for yrs.





